Skin cleansers and how they are used matter more than you think!
In my last post, I wrote about the importance of not over-cleansing and this is the perfect opportunity to delve into this topic in more detail.
The Stratum Corneum
The outermost layer of the skin, the stratum corneum, is made up of about 15-20 layers of dead skin cells called corneocytes, approximately 10-40 microns thick, filled with bundles of keratin protein, and held together with junctions called corneodesmosomes. These junctions are made up of a complex mix of mostly protein with some lipids. The corneocytes are flattened, and overlap each other. As the corneodesmosomes gradually break down, the corneocytes in the top-most layer are shed off and replaced by slightly newer corneocytes below. Besides being filled with protein, corneocytes also contain much of the chemical remnants of the formerly living skin cells. These chemicals, also referred to as “natural moisturizing factors”, along with the protein and lipids surrounding the corneocytes, form a relatively water resistant barrier that seals in the water content of living skin and body tissues underneath. Sebaceous glands secrete sebum, a complex mix of lipids and oils that soften the stratum corneum and provide additional water-proofing. Altogether, the stratum corneum has evolved to be a constantly renewing barrier to the outside environment that is very effective at keeping water content inside the body, and keeping bad stuff (pathogens, toxins, dirt) from entering the body…
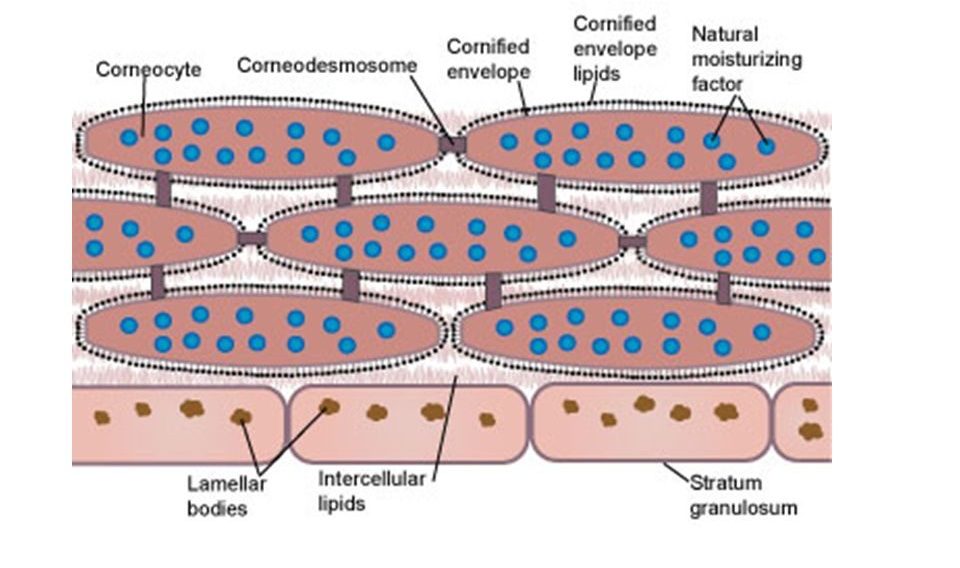
…That is, until it is compromised! And unfortunately, it is pretty easy to compromise the barrier function of the stratum corneum, because, as noted above, it is mainly held together with protein and lipids. All that is needed is to wash away the protein and lipid, and water can evaporate outwards, while allowing stuff to penetrate into the inner layers of the skin. And if things can enter the skin, the possibility of irritation or an allergic reaction goes up. The unfortunate thing is, people are doing that every time they lather up in a hot shower, especially if they happen to use a bar soap. So, how does that happen?
Acids, Bases and pH Level
You will often see the phrases “pH adjusted” or “pH balanced” used as marketing phrases on skin care products, but what does it mean, and what does it have to do with skin care? In order to talk about pH, we have to talk about what acids and bases are.
At room temperature (25°C) and regular atmospheric pressure, water dissociates at a very minimal level, as follows.
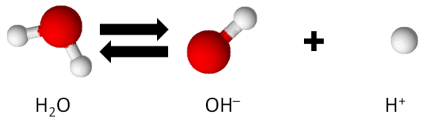
The concentration of hydrogen ions, H+, in water, denoted as [H+], under these conditions, is 1 x 10-7 mol/L.
All biochemical reactions take place in water. The Bronsted-Lowry theory of acids and bases applies to chemicals in water-based solution, and the theory defines acids and bases as follows:
- An acid is something that donates H+, i.e. it increases H+ concentration in the solution
- A base (also referred to as an alkaline substance) is something that accepts H+, thus decreasing H+ concentration in solution.
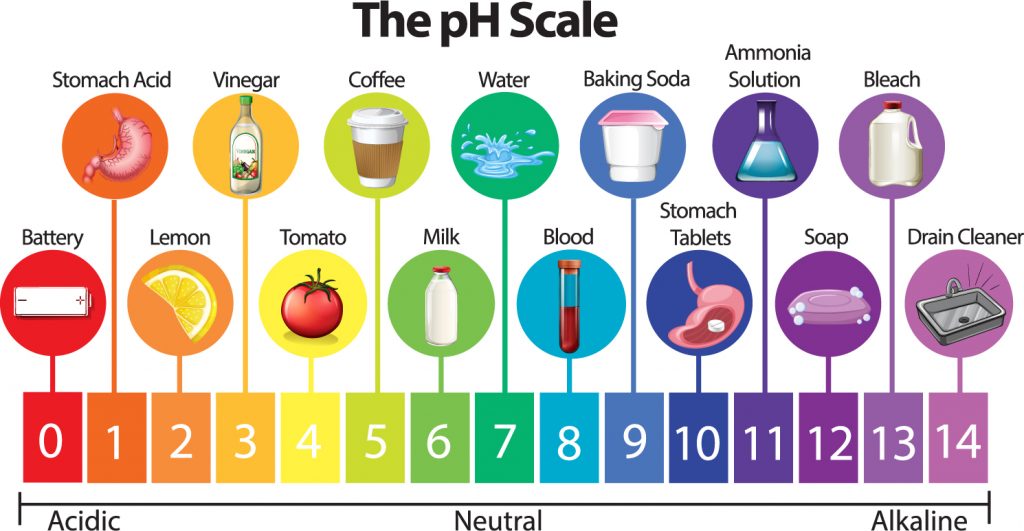
The pH scale is a negative logarithmic scale that allows a simple quantification of [H+] in solution. As noted above, at room temperature and regular atmospheric pressure, water has a hydrogen ion concentration of 1 x 10-7 mol/L . The exponent is -7; multiplying by -1 gives +7, so water has a pH = 7.
The pH scale runs from 0 to 14*. In water, acids will have a pH less than 7. Bases (also referred to as alkaline substances) will have a pH greater than 7. Water is neutral, as pH 7 falls right in the middle of the scale.
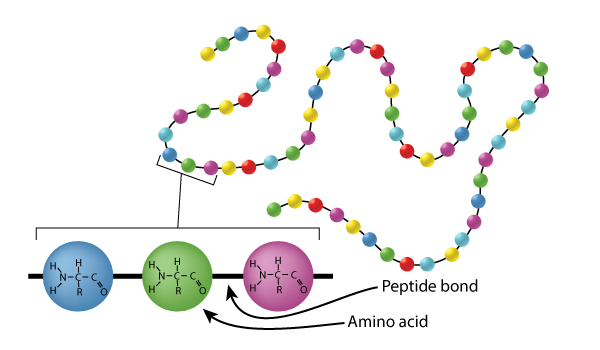
Proteins are made up of amino acids, strung together like beads on a string. The connecting bond between amino acids is an amide bond (sometimes called a peptide bond).
Amide bonds are pretty stable overall BUT it is possible to break them down using a strong acid or a strong base. Higher temperatures will speed things up.
Healthy skin is actually acidic, having a pH somewhere between 4.7 and 5.7. This acidity is maintained by the secretions from the sebaceous glands and the sweat glands, and it is believed to be a major defense against pathogens. This acidic environment is sometimes referred to as the “acid mantle”.
In addition to the acid mantle, there are “good” bacteria that colonize the skin, forming a microbiome. These bacteria live on the surface on the skin and colonize the sweat and sebaceous glands.
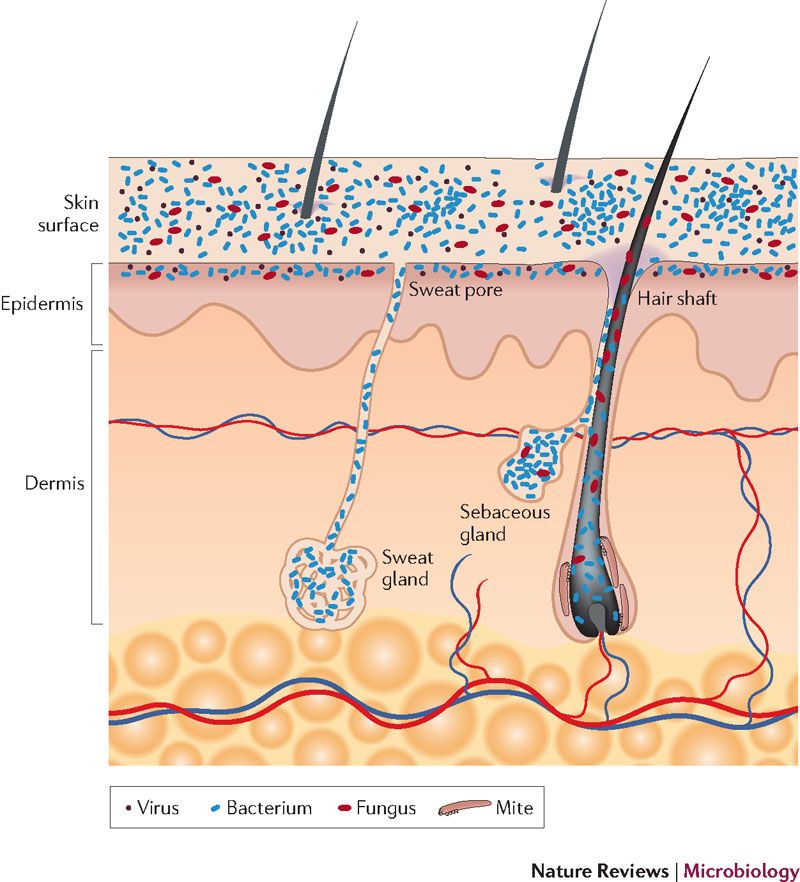
However, other than that, little is known about the skin’s microbiome, except that nearly all evidence points to its importance to overall skin health. It is believed that one way the skin’s microbiome offers protection against pathogens is that the “good” bacteria out-compete pathogenic bacteria and prevent them from taking hold. It is also believed that these bacteria help to train the body’s immune system at an early age to differentiate between something innocuous and something potential harmful. The “good” bacteria are adapted to live in the mildly acidic environment of healthy skin. Exposure to anything alkaline can damage not just the skin’s structural proteins, but also the skin’s microbiome, as it will also create an environment which cannot support the “good” bacteria.
Almost all traditional bar soaps are basic (alkaline) upon addition of water. Soaps are created by reacting a fatty acid (e.g. stearic acid) or a triglyceride, such as found in tallow, with a strong base, sodium hydroxide (lye). The product of this reaction is the sodium salt of the fat and glycerin (also called glycerol). The sodium salt of the fatty acid is the soap.
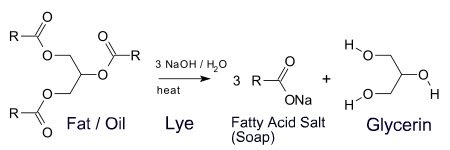
For example, reaction of stearic acid from tallow with sodium hydroxide will give sodium stearate. Sodium stearate is the salt of the conjugate base of stearic acid; upon addition of water, it will dissolve, releasing stearate ions which will act as a base, by drawing off free hydrogen ions from solution, and thus decreasing overall hydrogen ion concentration.
Using a portable pH meter, I tested a series of soaps**.
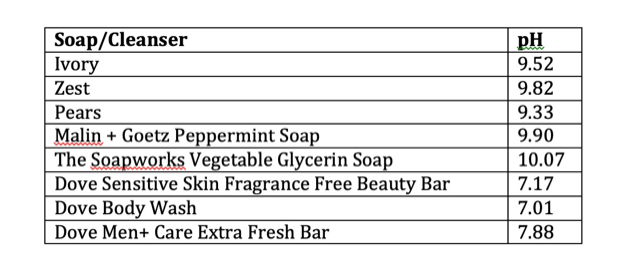
Traditional bar soaps like Ivory, Zest and Pears are made by saponification, so it’s not surprising to see that they are strongly alkaline, with an average of ~pH 9. This is why these soaps are very drying – they can break down protein, while the soap ingredients (sodium stearate/sodium palmate/sodium tallowate) solubilize proteins, lipids and the skin’s “good” bacteria, and wash everything away. Both hydrolysis and solubilization are increased in hot water, so washing with soap and hot water will really damage the outer layer of the skin. Not only that, but by raising the skin’s pH above 7, the environment needed to maintain the skin’s microbiome is destroyed.
Based on a completely unscientific survey – basically, me asking every person I met, what they use to clean themselves in the shower – not many people use traditional bar soaps these days. (Over the past few months, I only met one person who admitted to using Zest, and he immediately switched to Dove, when I explained the difference between various soaps and cleansers.) Nowadays, it seems that most people are choosing liquid body wash over solid bars, and buying niche brands which are usually marketed as more natural, milder or more luxurious.
Malin + Goetz is a good example of an upscale brand that has a “natural” slant to their marketing. Their bar soaps cost about $21 CAD and the peppermint soap is marketed as “gentle” and “suitable for sensitive skin”. However, with the fragrance content and pH =9.90, this is probably not a good idea for anyone with sensitive skin. If we take a look at the ingredient list, this is really just a traditional bar soap, made mainly of sodium palmate and sodium palm kernelate. It’s actually not much different from Ivory or Zest (which cost about $1 per bar), but it does smell nicer!
The Soapworks is a Canadian brand which has been around for a very long time. They are available at most health food stores as well as at Bulk Barn. They have gotten a boost in recent years because they are perceived as more natural, and also, the soaps are sold without any packaging, a major selling point for those trying to go zero waste. They have several different soaps, but based on a brief review of the ingredient lists, all appear to be traditional soaps. Being sensitive to scent, I decided to test the vegetable glycerin bar; according to the website, it is “perfect for sensitive skin and those with fragrance allergies”.

The ingredient list is as follows: sodium palmate, sodium palm kernelate, palm kernel acid, glycerin, and sodium chloride. This is a traditional soap formulation and it turned out to be the most strongly alkaline soap tested, with pH 10.07. The glycerin content, which is a humectant, would likely not be able to offset the drying quality of this soap.
As mentioned above, many people have switched to a liquid body wash, as there is the perception that these body washes are gentler on the skin. Most liquid body washes are based on synthetic detergents such as sodium lauryl sulphate, sodium laureth sulphate, ammonium lauryl sulphate (collectively known as “sulphates”) and/or sodium isethionate (a sulphonate). Because these ingredients are not conjugate bases and are not made via saponification (i.e. reaction with sodium hydroxide), cleansers based on these ingredients tend to be neutral in pH. I tested a few cleansers that are mainly based on synthetic detergents.
First up, I tested Dove Liquid Body Wash. Based on my completely unscientific survey, this is a very popular choice. It is composed almost entirely of synthetic detergents, the main one being cocamidopropyl betaine, along with emollients (soybean oil, stearic acid) and a humectant (glycerin) . It has a pH = 7.01. I would expect this one to be pretty gentle on skin but it is quite fragranced.
Dove Sensitive Skin Fragrance-Free Beauty Bar is a formulation that contains a blend of synthetic detergents and soap. In this case, the main synthetic detergent is sodium isethionate, which is a sulfonate. Sulfonates are not as powerful as sulfate detergents, in terms of their ability to solubilize oils. It’s slightly above neutral, with pH 7.17, and it’s unfragranced. This one is a good pick for people looking for an inexpensive, mild unscented soap.
Like many other brands, Dove has a men’s care line. I tested the Dove Men+ Care Extra Fresh bar, and was surprised to find it has a pH 7.88, which is definitely on the alkaline side… maybe they feel that men need more powerful cleaning than the regular Dove soaps?! It has a strong scent similar to Zest. With the fragrance and slightly alkaline nature, this is probably not a good choice for daily washes, especially hot showers.

Unfortunately, societal standards are such that we are expected to have a hot shower every day, and lather up as much as possible to be squeaky clean. People who exercise on a daily basis may end up having more than one shower in a day.
Polarity and Solubility
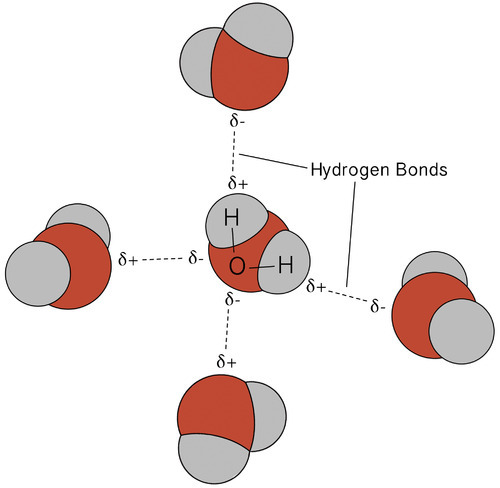
I started off by talking about pH level, but that is just one factor to consider. As mentioned above, all biochemical reactions take place in water. Water is a polar substance, meaning it has one end that negative (i.e. has a relative concentration of electrons, which carry a negative charge) and the other end is somewhat positive relative to the other end. Water molecules form transient hydrogen bonds between the negative end (the oxygen) of one water molecule and the positive end (the hydrogens) of another water molecule.
Oils, fats and waxes are made up mostly of long hydrocarbon chains. Charge distribution down these chains is relatively equal, meaning that these substances are non-polar. When oils and water are brought together, they will not mix, forming two separate layers; it takes energy to break apart the hydrogen bonds that hold together water molecules, energy that must be supplied somehow. One way to supply this energy is kinetic energy, i.e. mixing or shaking, but the layers will separate as soon as one stops mixing. Another way is to add heat energy, which increases molecular motion. However, the oil and water will again separate upon cooling.

There is a way to bring oil and water together: with amphiphilic chemicals, that have a polar group that can hang out with water and a non-polar group that can hang out with oils. Amphiphilic molecules occur naturally: phospholipids are one example, as they naturally form lipid bilayers that make up cell membranes (see panel D below). One important application of these dual-natured molecules is as cleansing agents. As seen in the diagram below, the polar or hydrophilic head group of these molecules likes to hang out with water, while the long hydrocarbon tail is non-polar and hydrophobic, and will stick to other non-polar substances, such as oils, fats, lipids and proteins.
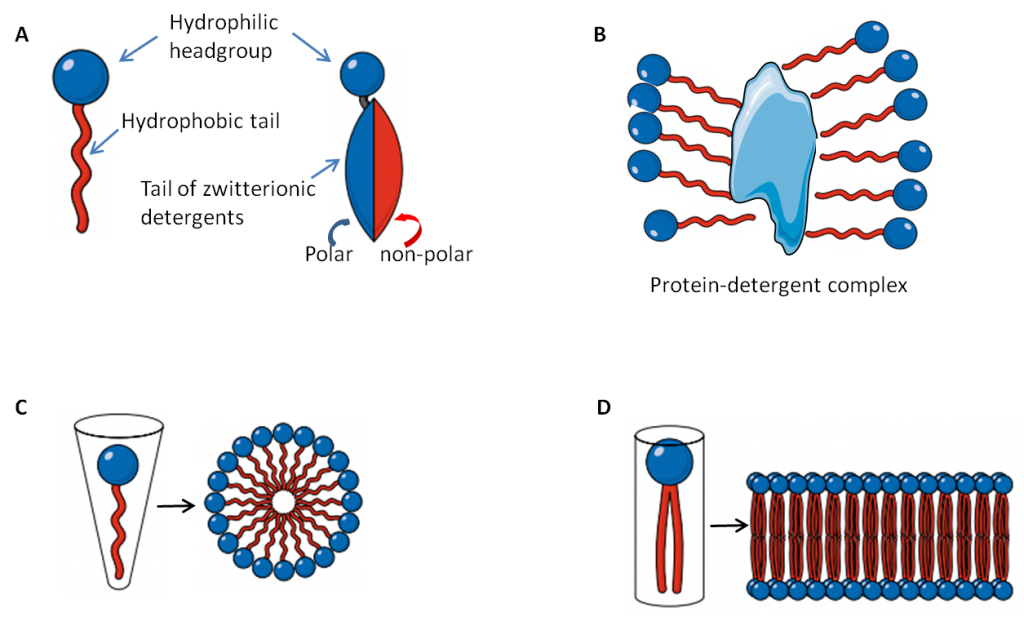
Soaps and detergents are amphiphilic. When applied to the skin, soaps and detergents solubilize proteins and lipids that make up the stratum corneum, stripping these valuable chemicals from the skin, as well as washing away all the bacteria that make up the skin’s microbiome. Solubilization is aided by hot water. So, even if a cleanser has a neutral pH, like the Dove Body Wash and Sensitive Skin bar, it’s still possible to overdo it, especially if lathering up in a hot shower.
As with everything else in life, moderation is the key. Use warm instead of hot water, and just apply soap/cleanser to the parts of your body that accumulate bacteria and sweat: underarms, groin, hands, feet. If you exercise, change up the shower schedule so that there is only one shower in a day and it comes after the workout. And an even more radical suggestion: if you don’t break a sweat on a daily basis, how about showering every other day instead of daily? You might find that less body moisturizer is needed overall, while dry flakes and irritations diminish over time. Body moisturizers, even good ones that contain lots of natural moisturizing factors, cannot come close to replacing all the naturally occurring components of the stratum corneum that are stripped away with daily washing and it definitely cannot replace the skin’s microbiome, so the best policy is to leave it as intact as possible! Your skin will thank you in the long run!
Notes:
*There are super acids and super bases which have pH values that fall outside of the 0-14 pH scale, but they are not something encountered in daily life.
**Soap solution was made with approximately 0.5 g of soap from each bar and 2 mL of distilled water. pH was tested using an Apera pH60F portable pH meter with a flat probe.