No matter what time of year, sun protection is the absolute best “anti-ageing” remedy there is.
I have written about sunscreens several times over the past decade and it’s a topic that needs to be updated regularly with the current consensus. As it has been a few years since my last update, it was high time for another review.
Although the effects are not immediately apparent, a daily sun protection routine will help stave off fine lines, wrinkles, freckles and sun spots. (When I say daily, that means year-round, even through fall and winter!) Even more important, sun protection is the best way to lower the risk of skin cancer, which has become the most common form of cancer. Given that skin cancer is on the rise, especially in those under 35, there’s just no reason to not use some form of sun protection anymore. Even if you have never used sun protection before, it’s never too late to start: sun protection gives your skin the chance to repair recent damage and helps to prevent or reduce additional damage.
We live in a time where we have a dizzying variety of sun protection options available to us. If you want to go old school, there’s hats, sunglasses and parasols. For those looking for more high tech solutions, there’s UPF-rated clothing, and various gadgets that monitor UV exposure.

However, for whatever reason, people tend to fixate on sunscreens. When it comes to sunscreens, there are a huge variety of formulations available, everything from balm sticks, to silicone-based dry-touch lotions, to dry mist sprays.
There are two types of active ingredients in topical sunscreens:
- Synthetic organic compounds (synthetic sunscreens), and
- Inorganic compounds (i.e. mineral oxides).
Synthetic sunscreens are carbon-based chemicals that have the ability to absorb and disperse the energy of UV radiation in the form of infra-red radiation (heat energy). The mineral oxides used for sunscreen, zinc oxide and titanium dioxide, absorb1 the energy of UV radiation, and re-emit this energy in the form of visible light. These are examples of fluorescence, a type of photoluminescence. In addition, these minerals scatter and reflect a small amount of incoming light.
Many people remain wary of synthetic sunscreens and turn towards ‘natural’ sunscreen products, which contain only minerals, namely, zinc oxide and/or titanium dioxide. Zinc oxide and titanium dioxide are very difficult to formulate into a cream base, and formulators usually have to use a high oil content in order to disperse the minerals, making many mineral-only sunscreens very thick and greasy-feeling on the skin. In addition, the formulator has to be careful to use adequate amounts of mineral oxide while ensuring the mineral is evenly dispersed. A good example is Honest Company, which ran into trouble when consumers complained that their SPF 30 sunscreen did not provide adequate protection. As it turns out, the sunscreen was reformulated to make it less greasy but at the same time, the zinc oxide content was lowered.
You may also see “all natural” sunscreens for sale at craft shows and farmer’s markets, but be careful: chances are the maker has not had it tested by an independent laboratory to certify the SPF rating. SPF testing is expensive and not within the budget of many independent crafters. Unfortunately, zinc oxide and titanium dioxide have a tendency to form clumps when trying to disperse into a cream or lotion. These clumps result in patchy, uneven coverage, thus defeating the purpose of the sunscreen.
Along with handmade sunblock creams containing mineral oxides, another natural-based trend is the use of coconut oil, and other vegetable oils, as a sunscreen on its own. This is a myth. Coconut oil and other vegetable oils provide only minimal sun protection as they do not contain chemicals that have the ability to adequately absorb the energy of UV radiation. It is estimated that coconut oil can only screen out about 20% of incoming UV radiation, and it does not provide broad spectrum protection against both UVA and UVB radiation. In comparison, a broad spectrum SPF 30 sunscreen protects against both UVA and UVB rays, and screens out ~97% of incoming UV radiation.
In recent years, there has been mounting concern over the effect of synthetic sunscreens on the organisms that live in and around aquatic environments2.

Photo credit: The Ocean Agency / XL Catlin Seaview Survey / Richard Vevers
There’s evidence suggesting that a number of synthetic sunscreen ingredients, including oxybenzone, benzophenone-1 and benzophenone-8, are toxic to coral reefs, and are compounding the effects of climate change, which is causing the oceans to heat up and become more acidic. There’s also concern that nanoparticles of the mineral oxides may also be damaging to aquatic ecosystems. All of these factors are leading to coral reefs bleaching and dying.
All of this is to say that, when it comes to sun protection, choose wisely:
- Check the weather prediction and the UV index before heading outside! UV protection is recommended for an index of 3 or higher.
- Avoid sun exposure between 10 am. and 3 p.m.
- Wear or carry your shade! Use a parasol or umbrella; wear a broad-brimmed hat and UV-rated sunglasses; wear tightly woven, opaque clothing that does not let sun shine through or UPF–rated clothing that covers arms and legs.
- Never rely on sunscreen products as your sole protection! If you must wear sunscreen, follow all the instructions carefully. Dermatologists3 recommend wearing a broad spectrum sunscreen with minimum SPF 30, and applying a “handful”, around 30 mL (2 tbsp) for the arms and legs. No matter what the value of the SPF is or the type of formulation, sunscreens wear off, and have to be reapplied every 2 hours, more often if sweating or going into the water. Sunscreens should be applied about 15 minutes before going into the sun to allow the product to dry down and form a uniform film on the skin. Unfortunately, most people never put on enough and invariably miss patches of skin.
Some suggested sunscreen picks, all of them mineral oxide-based. I recommend looking for ones that are labelled “reef safe”. However, be aware that many mineral-based sunscreens do contain nano-sized particles as it helps provide a more sheer/transparent formulation.

For a quick hit, check out the video I did for the Ontario Science Centre, on the Science of Sunscreens:
Questions or comments? Send me a message! 🙂
***
Notes:
- This is one section I had to update, as I had previously characterized mineral oxides as “physical blockers”, which is not scientifically accurate. I admit to some laziness here in not doing a bit more digging in reviewing the scientific literature. The vast majority of dermatology science publications and medical sources state that the mineral oxides work by physically blocking UV radiation from penetrating the skin, by scattering and reflecting it. This is likely based on the older use of zinc oxide as a thick white paste that covered up the skin, much like wearing a physical piece of clothing, and no one in the medical fields has bothered to elaborate, especially since it’s such a tidy explanation. This is a symptom of how scientists in different fields tend to work in silos without casting an eye to other disciplines, something of which I am certainly guilty of here, since I very nearly forgot my inorganic chemistry!
All of the crystalline forms of titanium dioxide have a very high refractive index (rutile form, refractive index n=~4), meaning that crystals of titanium dioxide can refract (bend) incoming light radiation. Zinc oxide also has a relatively high refractive index. Both minerals can scatter and reflect incoming light, which contributes to their bright white appearance.
Zinc and titanium are transition metals, which means they have a number of valence electrons. Valence electrons exist in the highest energy states allowed for that element, as described by the laws of quantum physics, and they give transition metals and their compounds all sorts of interesting characteristics.
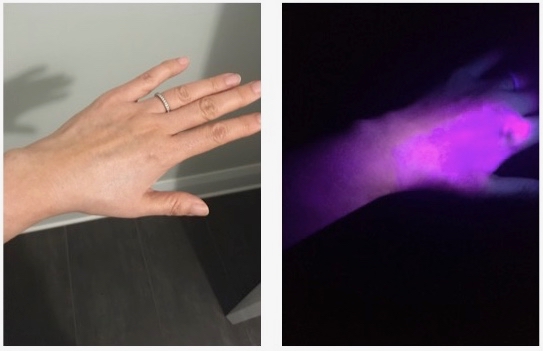
In addition, zinc oxide (ZnO) and titanium dioxide (TiO2) have excitation band gaps, wherein the valence electrons of Zn and Ti can be excited to a higher energy state. The band gap for TiO2 is 3.2-3.35 eV; the band gap for ZnO is 3.37 eV. This corresponds to an excitation peak of about 370 nm for TiO2 and 375 nm for ZnO, which is in the range of UVA radiation (UVA: 400-315 nm). Upon excitation with UVA, the valence electrons of these minerals are temporarily excited. Those electrons will almost immediately fall back down to ground state (the natural resting energy state), and when doing so, they emit a portion of that excitation energy as visible light radiation, a phenomenon known as fluorescence. The remaining energy likely is dispersed as infrared radiation (heat).
Upon excitation, both minerals are weakly fluorescent: TiO2 emits the majority of its excitation energy in the form of violet/blue light, with a weak peak at around 421 nm, and ZnO emits a low intensity cyan/green light, around 500-530 nm. Under daylight conditions, the eye does not perceive a blue glow coming off the skin (fluorescence is most visible to the naked eye in a dark environment). Instead, the eye perceives whiteness, which contributes to the ghostly appearance of mineral oxide creams once applied to the skin. (Skin-toned tints using pigments, especially for SPF 30 or lower, help to mask the white appearance of ZnO and TiO2.)
Selected references:
Cole, C. et al. “Metal oxide sunscreens protect skin by absorption, not by reflection or scattering” Photoderm Photoimmunol Photomed. 2016 Jan; 32(1):5-10. https://doi.org/10.1111/phpp.12214
Trivedi, M. et al. “Titanium Dioxide in Sunscreen.” July 26, 2017. Intechopen. DOI: 10.5772/intechopen.68886
Ji, J., Colosimo, A., Anwand, W. et al. ZnO Luminescence and scintillation studied via photoexcitation, X-ray excitation and gamma-induced positron spectroscopy. Sci Rep 6, 31238 (2016). https://doi.org/10.1038/srep31238
Hahm, J. “Zinc oxide nanomaterials for biomedical fluorescence detection.” J Nanosci Nanotechnol. 2014 Jan; 14(1): 475–486.
2. To read more about what’s happening with coral reefs around the world, see:
https://oceanservice.noaa.gov/facts/coral_bleach.html
https://www.theguardian.com/environment/coral
For more information on suspected sunscreen ingredients, see: https://oceanservice.noaa.gov/news/sunscreen-corals.html
3. Check the Canadian Dermatology Association for additional information on sun protection, guidelines for sunscreen use, and skin care recommendations.

